Cosmology - Intro to Nucleosynthesis
OPENING QUESTIONS: Work with your group to determine six (6) significant events from (and including) Planck time to + 1 billion years.
Write each item on a slip of paper found on the back table (Please write in one or two words only, nice and big using the sharpies provided)
OBJECTIVE: I will work with my team and then the class to develop a list of 6 events that you feel are MOST relevant after the Big Bang.
I will be able to describe the basis of stellar fusion (nucleosynthesis) during today's class
WORDS FOR TODAY:
- Planck Time
- Proton (a non-fundamental particle, contains u-u-d quarks, has +1 charge)
- Electron (a fundamental particle of nature, has -1 charge)
- Neutron (a non-fundamental particle, contains d-d-u quarks)
- Up Quark (a fundamental particle of nature, has + 2/3 charge)
- Down Quark ((a fundamental particle of nature, has -1/3 charge)
- Strong Force (between quarks)
- Weak Force (not exactly a force)
- Electromagnetic force (between protons & neutrons)
- Gravity (between objects with mass)
- Nucleosynthesis (creating new atoms)
- Fusion (atoms smashing together - a more general term for nucleosynthesis)
- isotope (an atom with the same number of protons but different numbers of neutrons)
WORK O' THE DAY:
Let's work to get a class consensus-- up to the whiteboard please (and bring your slips)
═══════════════════════════
Now let's get a wee bit technical.
Hydrogen almost never has a neutron in its nucleus. However that doesn't mean that hydrogen can't have a neutron in its nucleus. As a matter of fact it can. That is a special type of hydrogen so we call it deuterium (meaning two atomic particles). In fact, in much rarer instances, hydrogen can have 2 neutrons in its nucleus, we call that... (wait for it) tritium.
Note: hydrogen is the ONLY atom on the periodic table where we give names to the different isotopes. That's because they are critical to understanding fusion reactions in the sun and other stars.
Work with your team to determine how we might represent those two flavors (the scientific term is 'isotopes') of hydrogen.
atom
Notice that the superscript shows the total number of particles (p+ and n0) in the nucleus
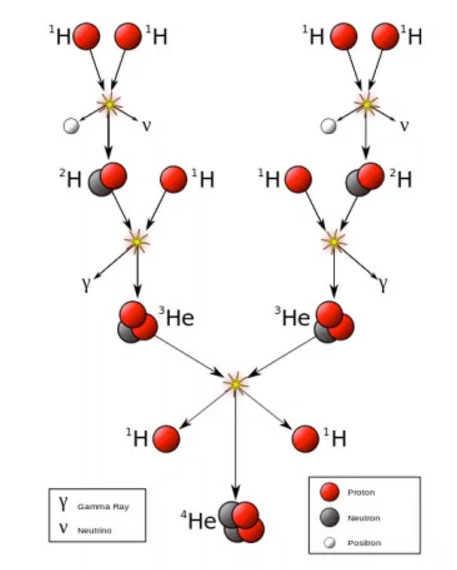
With that in mind, please work with your team to sketch the process where hydrogen is fused into helium in our sun (and other stars) in the process we call 'nucleosynthesis')
Did you do something like this? (Most of us do!) <see whiteboard>
Hint: where does the energy come from?
Here's an interesting learning target that I learned from the NGSS standards: The total number of nucleons in a nuclear reaction is conserved.
Huh?
Let's discuss.
I *did* say we weren't going to go into the weak force, but,. well, ya know, I thought I'd have us all take a gander at THIS, and we can decide whether we want to move on or not....